

by Chris Woodford . Last updated: July 26, 2023.
S now, sea, cloud—it's not often you see what look like the three main states of matter (solid, liquid, and gas) in the same place, at the same time. But I got lucky one chilly day earlier this year walking on the beach just after a snowstorm. The clouds (aerosols, slowly forming from invisible water vapor) were still heavy with rain waiting to fall, there was a dusting of snow (solid water) on the beach, and the ocean (liquid water) was licking in and out, in and out. There, right in front of my eyes, was water in its three states, all together at once.
Now you can see three states of different substances any time you like. Open the door of your refrigerator and you'll see all kinds of liquids chilling in jars, solid lumps of vegetables and cheese, and the whole chiller cabinet bursting with invisible gases—oxygen, nitrogen, carbon dioxide, and the various other, lesser-known ones such as argon that make up the air around us. But it's not often you see three states of the same substance all together at once. Ever wondered why? Let's take a closer look!
Photo: Water in its three states? Snow (solid water) on the beach, liquid water in the ocean, and water vapor (water cooling from gaseous form into water droplets and ice crystals, slowly forming into visible clouds). What's the difference between water vapor and steam? Steam is water in the form of a hot gas made by boiling water, whereas water vapor is water in a gas form at any temperature—it could be cold water vapor made from liquid water by lowering the pressure. Although clouds might look like simple white gases, they're actually examples of aerosols (liquids or solids dispersed in gases—so either water drops or ice crystals smeared out through a big mass of air).
What's the difference between a solid, a liquid, and a gas? You might think it's just a matter of temperature, but there's more to it than that.
In solids , atoms are bonded fairly firmly together, though they do move about a bit. You don't need to put a solid in a container; it stays where it is because its atoms are locked tightly into a definite shape that, ordinarily, doesn't change. If a solid is reasonably soft and you press it, you can make it change shape by pushing its atoms into new positions.
Heat a solid enough and you'll give its atoms enough energy to break apart, forming a liquid. In liquids , the atoms are more randomly arranged and a little bit further apart (but not all that much). The forces between them are weaker and they can jiggle about and flow past one another quite easily. That's why liquids pour. Take enough heat away from a liquid and the atoms will slow down until they form a solid. Add some more heat and some of the atoms can escape from it to form a gas.
Gases have much more randomly arranged atoms than either liquids or solids. The forces between the atoms are very weak, so the atoms can speed around freely with lots of energy. A liquid can flow, but a gas goes one better and expands to fill all the space available to it. If you squeeze a gas really hard or take heat away from it, its molecules have to huddle together. Pretty soon they're bonding to form a liquid. Keep squeezing or cooling and you'll lock them together tightly to make a solid.
Solids, liquids, and gases are all made of atoms—but how those atoms are arranged is different in each case.
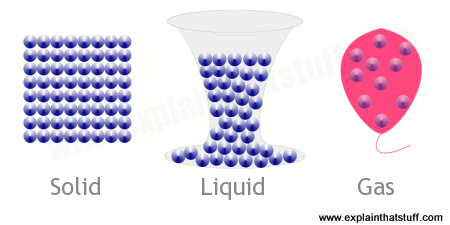
Solids (left) are more dense than liquids: they have more atoms packed into the same space. The atoms are tightly packed together and stay in shape all by themselves, though they do move about on the spot.
Liquids (middle) are usually less dense than solids but more dense than gases. Their atoms can move around much more, so they need a container to keep them in place (but an open container is usually okay for short periods of time). Although diagrams in books often exaggerate the spacing, it's important to note that the atoms in liquids are almost as close together as they are in solids.
Gases (right) are even less dense than liquids. Their atoms are much more spread out and go where they please, so they need a completely sealed container (such as a rubber balloon) to keep them in place.
Sponsored linksYou can change any substance from a solid to a liquid or gas, or back again, just by changing its pressure and/or temperature, but that's not immediately obvious to us in a world where the temperature and pressure don't change much at all. On Earth, temperatures broadly vary from about −30°C to +30°C or (−70°F to +90°F)—which seems a huge variation to a warm-blooded human but doesn't worry a block of iron very much. Air pressures go up and down too. Air pressure is often measured in units called atmospheres, where normal pressure measures 1 atmosphere (equal to 101 kPa in proper, scientific units of pressure, known as kilopascals). The lowest air pressures on Earth's surface (encountered during things like tornadoes) are about 0.8 atmospheres (85 kPa), while the highest reach about 1.1 atmospheres (110 kPa). Again, the difference between those numbers seems extreme and dramatic to us, because it signals huge changes in the weather, but it doesn't change things nearly enough to make any difference to a block of wood or a slab of granite.
Artwork: Changing states: You can change a solid into a liquid by melting it and then change the liquid into a gas by evaporation. Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing. But, given the right temperature and pressure conditions, you can also change solids directly to and from gases by sublimation and deposition. The processes shown by each pair of arrows are exact opposites of one another.
In the narrow band of temperature and pressure we live in, most of the things we encounter are either solid, liquid, or gas—and stay that way. Water is the obvious exception. When I said I saw snow, water, and ice together in the same place at the same time on a beach in winter, I was cheating—ever so slightly! The snow on the beach is at a slightly lower temperature than the water in the ocean, while the water vapor in the sky is at a much lower pressure. So in fact the three states of water coexist in my photo because they're in quite different conditions on land, ocean, and sky. Compared to water, other substances need much more extreme changes of temperature or pressure to change their state. If you want to change something like a block of iron from a solid into a liquid, you're looking at heating to temperatures of about 1500°C (2750°F). On the other hand, to change an everyday gas such as nitrogen into a liquid, you'd need to cool it down below about −200°C (−320°F).

Photo: Nitrogen makes up most of the air all around us and it's usually an invisible gas. If you cool it down enough, you can condense it into liquid nitrogen, which has many scientific and medical uses. In this photo, a technician is filling a tank with extremely cold liquid nitrogen, so he needs to wear gloves to protect his hands. Some of the liquid evaporates and turns back into a smoky vapor before disappearing into the air. Note the frost on the pipes and valves behind him! Photo by Stephani Barge courtesy of US Air Force and DVIDS.
Another way to understand solids, liquids, and gases is by thinking about the energy they contain. A balloon full of gas has molecules dashing about inside it, smashing repeatedly into the rubber walls and pressing them outward. Balloons stay up because the force of the gas molecules pushing against the inner surface of the rubber exerts a pressure that's equal to the pressure of the air molecules pushing on the rubber from outside.
Artwork: A balloon stays inflated because gas molecules repeatedly collide with its thin rubber wall, pressing it outward. The force of these collisions is exactly balanced by the elasticity (stretchiness) of the rubber.
Since there are molecules inside the balloon moving about, we must have kinetic energy inside the balloon too—because the molecules have both mass and velocity. Heat a balloon up and you give the molecules more energy: they absorb the heat you supply and move about a bit faster (with more velocity), so they crash into the walls harder and exert more pressure. That's why heating a balloon makes it inflate. Similarly, cool a balloon down (by putting it in the refrigerator) and you rob the molecules inside of some of their energy. That means they crash less energetically into the balloon walls and exert less pressure, so the balloon goes down. Understanding how solids, liquids, and gases behave in terms of the heat their moving molecules possess is known as the kinetic theory of matter .
What if you cool down a balloon—and keep cooling? Suppose you fill your balloon with steam to start with. Cool it for a while and you'd get a balloon with a bit of water inside, then a balloon frozen with ice. If you keep on cooling, you take more and more energy from the molecules inside. Even the atoms or molecules in a solid do move about a little bit, but they're like people running on the spot: they oscillate more or less around a fixed position. The more you cool a solid, the less the atoms or molecules move.
Could you reach a point where the atoms or molecules stopped moving altogether? In theory, yes. If you figure out the numbers, you'd need to cool down to −273.15°C (−459°F), which scientists describe as the lowest theoretically possible temperature or absolute zero . In practice, it looks impossible we could ever cool things down this much, though researchers have got pretty close. You can read more in a PBS/NOVA website all about the quest to reach absolute zero.
Animation: As we cool toward absolute zero, the atoms or molecules inside a substance slow down. At absolute zero, they stop moving altogether.
If absolute zero really is the lowest limit of temperature, why don't we readjust our temperature scales starting from there as our zero point? That's what British physicist William Thomson (1824–1907, also known as Lord Kelvin) proposed in 1848. The modern Kelvin scale (also called the absolute scale ) numbers up from 0K in temperature units the same size as degrees Celsius, so the freezing point of water (0°C) becomes 273K and the boiling point of water (100°C) is 373K. Temperatures on the Kelvin scale are written without a degree (°) sign.
A solid lump of iron is much heavier than a glass of water the same size, while a balloon that's many times bigger seems to weigh nothing at all. Some solids, such as rubber, are very stretchy: you can pull a rubber band to two or three times its length and it will snap straight back to its original length when you let go. Other solids (like glass or china) smash if you drop them. So what makes some solids different from others? What makes solids work differently from liquids and gases?

Photo: Solid properties: All these things are solids, but they have very different physical properties. Steel (can, left) is strong, resists heat well, conducts electricity, and melts at a high temperature. Rubber (tennis ball, top) is stiff, stretchy, melts at a low temperature, and doesn't conduct electricity. Plastic (bottle, right) is fairly strong, squashes when you push it but doesn't usually return to its original shape, melts at a low temperature, and doesn't conduct electricity or heat. Artificial sponge (top right) is similar to rubber but much weaker and less durable.
We describe all the ways in which a particular substance behaves as its properties . The changes it goes through when we push it or pull it, put electricity through it, heat it, or try to smash it with a hammer are called its physical properties. How it reacts with other substances are called its chemical properties.
We can understand the physical properties of a solid, liquid, or gas by looking at what the atoms or molecules are doing inside it. For example, what makes rubber stretchy? The molecules are made from long chains of atoms and these are joined to one another by weaker bonds called cross-links. When you stretch rubber, you can pull these links apart. When you let go, the links are strong enough to pull the chains back together again. Here's another example: Why are solids so heavy? Because there are so many atoms packed into a very small space. That also explains why most solids are hard and don't easily change their shape. Inside a solid, the atoms are packed tightly together and it's hard to make them move past one another. Because the atoms are so reluctant to move, a solid has trouble changing its shape. It turns out that we can explain all the properties of solids, liquids, and gases by looking at what's happening to the atoms or molecules inside them. This is called materials science.
If you heat a liquid, sooner or later you get a gas—but what happens if you keep heating? Eventually you produce a fourth state of matter called a plasma , in which the gas molecules not only separate from one another but break apart into their subatomic components—electrons and ions (in this case, atoms missing electrons). Plasmas are used in plasma TVs (now-obsolete but still interesting), among other things.

Photo: A plasma "sphere" is a completely sealed glass bowl (or, in this case, cylinder) containing hot, ionized gas produced with the help of electricity. If you place your hands on the glass, they attract the free electrons in the plasma, so it swirls about in response to your touch! Photo by John Suits courtesy of US Navy and Wikimedia Commons.
I've just broken the "bad" news that there are four states of matter, not three. But is that the end of the story? Nope! There are a few others that exist only under extreme conditions. The best known of these are called Bose-Einstein condensates (in honor of physicists Albert Einstein and Satyendra Nath Bose). They're formed when special gases made of bosons (some of the fundamental particles from which atoms are made) are cooled to temperatures near absolute zero and condense into a new state of matter. There are some other states of matter too, but you won't meet them in your refrigerator—and I won't go into them here. There are also various "hybrid" states such as liquid crystals, which are part liquid and part crystalline solid. When we say there are three or four states of matter, you can see it's really quite a simplification!

Artwork: How a Bose-Einstein condensate forms. Atoms condense from the red, yellow, and green areas into the denser blue and white areas. Photo by NIST (National Institute of Standards and Technology) courtesy of Wikimedia Commons.